Green Antioxidants: Synthesis and Scavenging Activity of Coumarin-Thiadiazoles as Potential Antioxidants Complemented by Molecular Modeling Studies
DOI:
https://doi.org/10.5530/fra.2016.2.7Keywords:
Coumarins, Hartree–Fock, Scavenging activities, Antioxidant, PicrylhydrazylAbstract
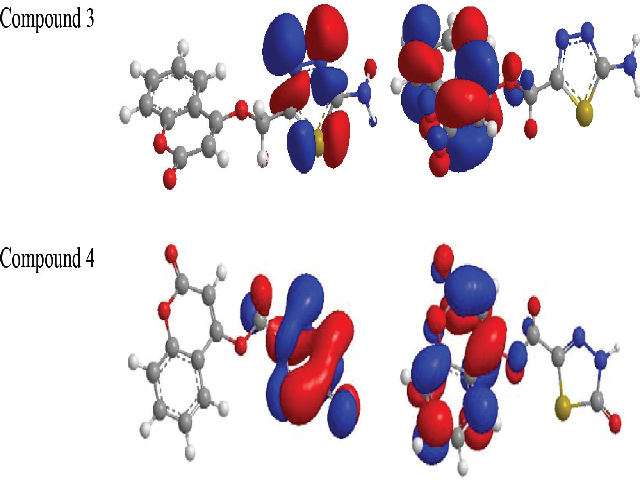
Introduction: Coumarin is a natural product compound known for its medicinal properties. Methods: The rational design of 4-hydroxycoumarin functionalized thiadiazoles (3 and 4) was synthesized with tailor-made antioxidant properties. The strategy involves intra-molecular cyclization of 2-(coumarin-4-yloxy) acetic acid under reflux conditions. Antioxidant activity of synthesized compounds were performed using various in vitro assays against 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical and hydrogen peroxide (H2O2) scavenging. Results: The results revealed that tested compounds 3 and 4 are much higher than well-known antioxidant compound naming ascorbic acid. They showed good scavenging capacity against DPPH and hydroxyl radicals. Ab initio HF (Hartree–Fock) with 3-21G basis set for the title compounds demonstrated the correlation of scavenging activities and theoretical parameters (such as, dipole-moment, ionization-potential, electron-affinity and highest occupied molecular orbital). Conclusion: In conclusion Coumarins were successfully synthesized and were evaluated as antioxidants by DPPH and hydrogen peroxide assays and they were indicated that they had good scavenging activities. The synthesized antioxidants were investigated theoretically and also some quantum chemical parameters were calculated.
Downloads
Metrics