A comparative analysis of superoxide dismutase activity levels in gingiva and gingival crevicular fluid in patients with chronic periodontitis and periodontally healthy controls
DOI:
https://doi.org/10.5530/ax.2012.4.4Keywords:
Antioxidant mechanism, Chronic periodontitis, Gingiva, Gingival crevicular fluid, Superoxide dismutase, superoxideAbstract
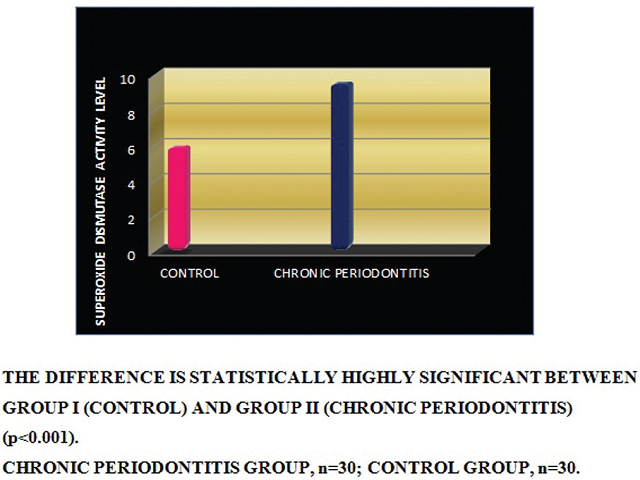
Background and Objectives: Superoxide dismutase (SOD), a metalloenzyme is a powerful antioxidant in the body that acts against superoxide, released in inflammatory pathways and causes extracellular structure degradation. In this study, SOD activities in gingiva and gingival crevicular fluid (GCF) from patients with chronic periodontitis (CP) and periodontally healthy controls were compared. Materials and Methods: Thirty patients, involving teeth with moderateto- severe periodontal breakdown and ≥5 mm pockets that required full-thickness flap surgery in the maxillary quadrants, and in controls, thirty patients with teeth scheduled for extraction for reasons other than periodontal destruction were studied. After clinical measurements, GCF samples were collected. Tissue samples were harvested from the same teeth, during flap operation in CP patients and immediately after tooth extraction in controls. SOD activities were spectrophotometrically assayed. The results were statistically analyzed. Results: Gingival SOD activity was significantly higher in the CP group than in the controls (p<0.001). Significant difference was found in GCF SOD activity between the groups (p = 0.012). Correlations between gingival and GCF SOD were statistically highly significant in CP and control groups (p = 0.00). Conclusion: In chronic periodontitis, superoxide dismutase activity seems to increase in gingiva, probably as a result of a higher need for superoxide dismutase activity and protection in gingiva in CP, while a lower activity seen in GCF which could be due to the low amounts of superoxide dismutase in the extracellular fluid. The strong correlation between the gingival and GCF SOD activities suggests distinct actions of these superoxide dismutases.
Downloads
Metrics